Mastering Restaurant Sanitizing: Best Practices for Kitchens and Dining Areas
Master restaurant sanitizing with clear steps for kitchens and dining areas. Learn methods, checklists, and procedures to keep your team safe and...
FDA, PCQI, FSMA… What do these alphabet soup acronyms have in common?
If you’re the owner of an FDA-regulated food processing facility, or you work at one, a PCQI — Preventive Controls Qualified Individual — will be your key to effective food safety and FSMA compliance.
At a high level, your PCQI-certified food safety team member will help reduce the potentially daunting nature of inspections while increasing the confidence in and success of your audits.
Day to day, PCQIs develop and oversee your food safety plan, validate preventive controls, and review records for implementation and effectiveness. All of these things work toward ensuring your facility meets FDA requirements and produces safe, high-quality food products.
A PCQI is essential in food facilities regulated by the FDA, ensuring compliance with the Food Safety Modernization Act (FSMA) and maintaining high food safety standards.
PCQIs develop and oversee the facility's food safety plan, validate preventive controls, review implementation records, train employees on food safety procedures, conduct risk assessments, and stay updated on FDA regulations.
Having a PCQI on your team can help improve food safety and quality, and enhance the facility's brand reputation as well as increase consumer trust.
PCQI training can be completed through courses recognized by the FDA, including instructor-led training, online self-paced courses, and blended learning.
According to the FSMA, food facilities must have a written food safety plan developed by a PCQI. This plan must include hazard analysis, preventive controls, supply chain programs, and recall plans, with ongoing compliance and regular updates.
PCQIs can help facilitate the process of obtaining third-party certifications like SQF, as many certification standards align with FSMA requirements.
The PCQI plays a critical role in preparing for FDA inspections by regularly reviewing the food safety plan, verifying preventive controls, maintaining accurate records, and ensuring team members follow procedures.
Mock inspections and audits, along with continuous improvement practices, are vital for maintaining compliance, identifying potential issues, and ensuring the facility remains inspection-ready and committed to producing safe, high-quality food products.
FoodDocs' food safety management software can help PCQIs implement their food safety plan and maintain compliance with it!
In the context of food safety, PCQI stands for Preventive Controls Qualified Individual.
A Preventive Controls Qualified Individual is a key role in the food industry, responsible for ensuring that food facilities comply with FDA regulations and maintain the highest standards of food safety.
The PCQI is a specially trained individual who has:
The primary responsibility of a PCQI is to develop and oversee the facility's Food Safety Management System. This system includes a critical document that outlines the steps that food manufacturers' takes to ensure the safety of the food products they manufacture, process, pack, or hold. The PCQI must have a deep understanding of the potential hazards associated with the facility's products and processes, and must design preventive controls to mitigate these risks.
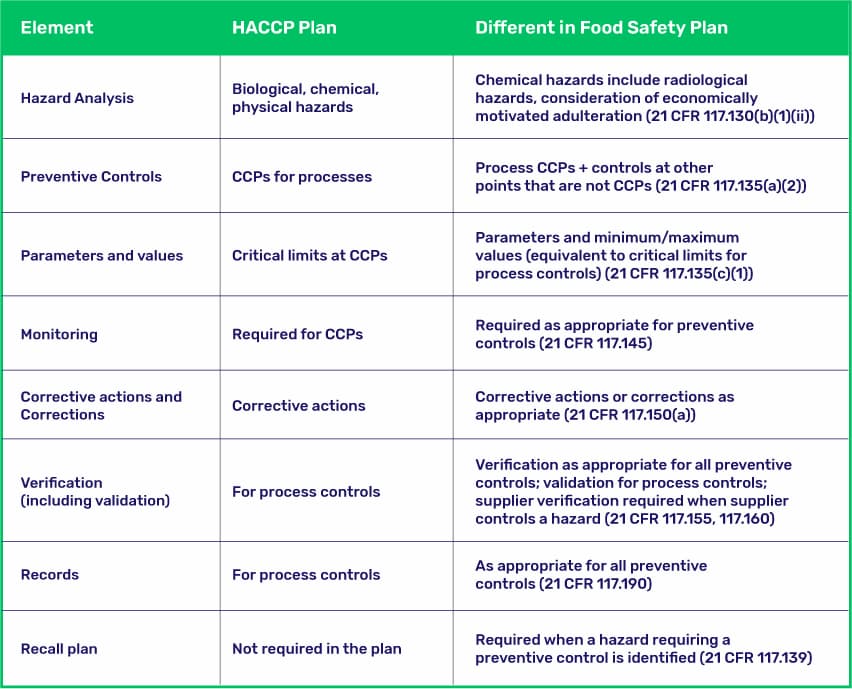
In addition to developing the food safety plan, the PCQI is responsible for validating the effectiveness of the preventive controls. This involves collecting and analyzing data to ensure that the controls are working as intended and are effectively preventing potential hazards.
The PCQI must also review records regularly to verify that the food safety plan is being followed and that the preventive controls remain effective over time.
While both PCQIs and HACCP (Hazard Analysis and Critical Control Points) focus on food safety, there are some key differences between the two.
HACCP is a voluntary program that has been widely used in the food industry for decades, focusing on identifying and controlling critical control points in the manufacturing process. Although, industry standards often require it.
HACCP Plans identify critical control points within the process, while a PCQI’s food safety plan should identify both process control points and CCPs determined by the facility.
From a regulatory perspective, the PCQI role was created as part of the Food Safety Modernization Act (FSMA), which shifted the focus from responding to foodborne illness outbreaks to preventing them from occurring in the first place. PCQIs take a broader view of food safety, looking at the entire food safety system rather than just critical control points.
Another key difference is that the FDA mandates PCQI training for most food companies. The PCQI training is also more comprehensive and, in addition to traditional HACCP principles, covers:
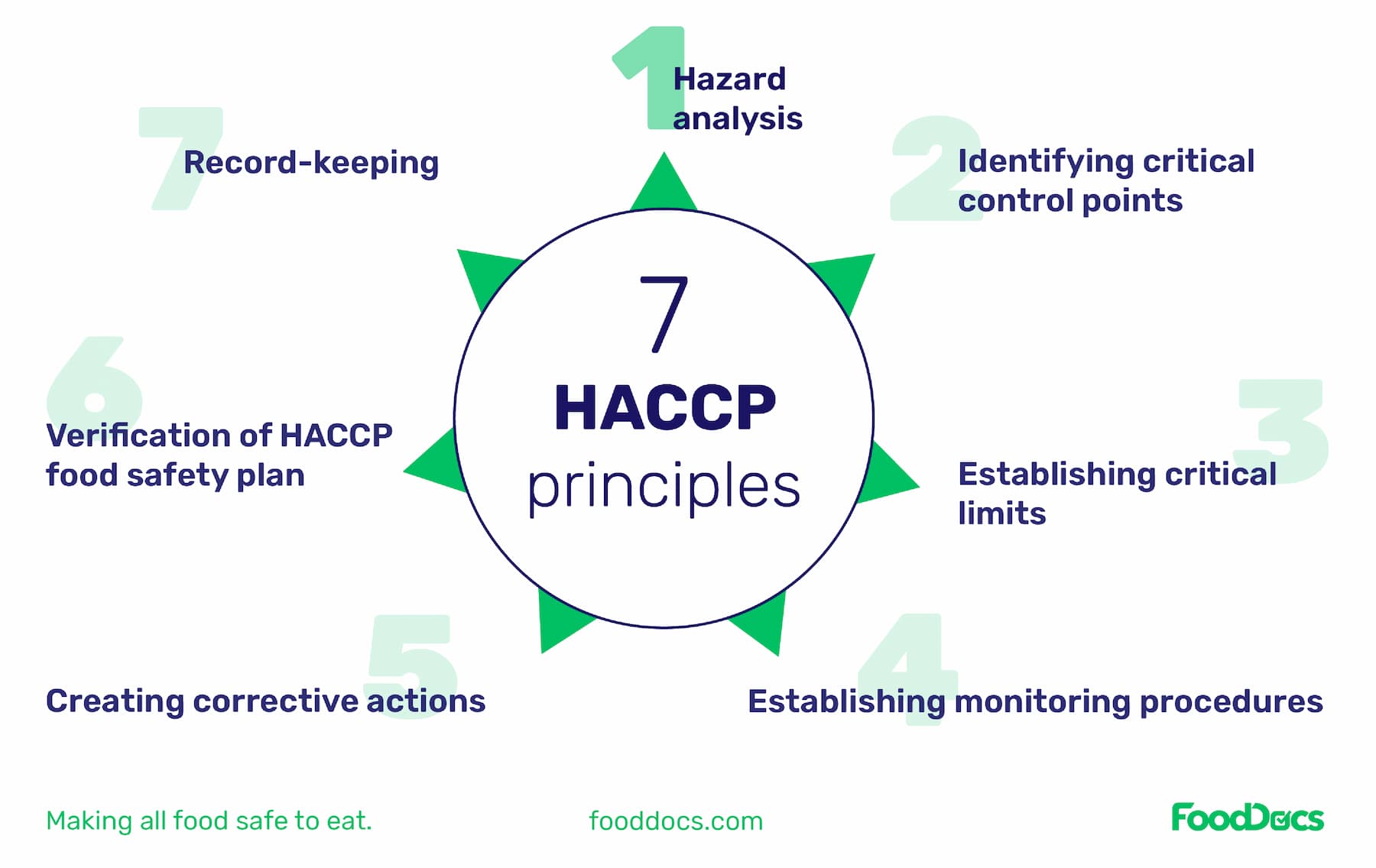
Even if you have other food safety certifications such as HACCP, the FDA will generally "assess the adequacy of a facility’s food safety plan rather than an individual’s documented qualifications. Deficiencies in the food safety plan indicate that a PCQI may need additional training specific to the rule, irrespective of documented training and experience."
Having a PCQI on staff is crucial for any food facility that wants to stay compliant with FDA regulations and maintain a high standard of food safety.
Having a PCQI oversee your food safety plan helps food facilities to avoid costly violations and, worst case, shutdowns. Investing in PCQIs demonstrates a commitment to food safety because it shows the state and its inspectors just how much your facility takes responsibility for protecting public health.
Your PCQI will help to improve food safety and quality. The PCQI is trained to identify, control, and prevent potential hazards in the food production process. This includes biological, chemical, and physical hazards that could contaminate food and cause illness. PCQIs can also establish monitoring and verification procedures to ensure that your business produces food at a high quality standard, consistently.
Finally, having a PCQI on staff can enhance your facility's brand reputation and send a strong message to customers that your business prioritizes not just food safety, but their safety. This is especially important nowadays because conscious consumers are increasingly concerned about their food’s safety and quality.
Not to mention, adding a PCQI to your team roster can increase brand trust and loyalty while giving you a competitive advantage against businesses that don’t yet have one or make known that they have one.
If you recall from earlier in this article, we mentioned that one way you can become a PCQI is through gaining equivalent knowledge through job experience.
With that mind, there’s technically no accredited certification Preventive Controls Qualified Individuals program. However, there are a handful of solid online PCQI training courses, some of which provide you with a certificate upon successful completion.
What the FDA does specify is this:
“[A] PCQI is a qualified individual who has successfully completed training in the development and application of risk-based preventive controls at least equivalent to that received under a standardized curriculum recognized as adequate by FDA or is otherwise qualified through job experience to develop and apply a food safety system… However, the rule does not require any specific certifications, including certification by the Food Safety Preventive Controls Alliance (FSPCA). An individual may voluntarily choose to attend the PCQI training provided through the FSPCA, but this is not mandatory.”
Generally, to become a PCQI, an individual must complete a specialized training course that the FDA has approved. These courses are offered by a variety of organizations, including universities, trade associations, and private training companies.
The FSPCA offers one of the most well-known PCQI courses in three different formats, depending on your schedule and learning styles:
You can check out the public participant manual yourself for more detail. But this is the type and progression of content that you can expect in a PCQI training course:
|
Day 1 |
Chapter 1 |
Introduction to Course and Preventive Controls |
|
Chapter 2 |
Food Safety Plan Overview |
|
|
Chapter 3 |
Good Manufacturing Practices and Other Prerequisite |
|
|
Chapter 4 |
Biological Food Safety Hazards |
|
|
Chapter 5 |
Chemical, Physical and Economically Motivated Food Safety Hazards |
|
|
Chapter 6 |
Preliminary Steps in Developing a Food Safety Plan |
|
|
Chapter 7 |
Resources for Preparing Food Safety Plans |
|
|
Day 2 |
Chapter 8 |
Hazard Analysis and Preventive Controls Determination |
|
Chapter 9 |
Process Preventive Controls |
|
|
Chapter 10 |
Food Allergen Preventive Controls |
|
|
Chapter 11 |
Sanitation Preventive Controls |
|
|
Chapter 12 |
Supply‐chain Preventive Controls |
|
|
Day 3 |
Chapter 13 |
Verification and Validation Procedures |
|
Chapter 14 |
Recordkeeping Requirements and Procedures |
|
|
Chapter 15 |
Recall Plan |
|
|
Chapter 16 |
Regulation Overview – cGMP, Hazard Analysis, and Risk‐Based Preventive Controls for Human Food |
To accommodate different learning preferences and schedules, PCQI training is available in various formats:
In addition to the FSPCA’s PCQI training course, others include:
Remember, there’s no accredited PCQI certification. But the cost of PCQI training ranges depending on who’s offering the training and in what format. For example, the Food Safety & Quality Services course is priced at $699, Registrar Corp and Zosi’s are both $799, AIB International’s is $588, ImEPIK’s is $775.
Short answer, no. If you successfully complete an FDA-recognized PCQI training course, there’s no specified expiration or PCQI renewal date outlined in the Preventive Controls for Human Food rule. It’s totally up to the individual and their place of work to determine if and when it’s necessary to refresh their PCQI knowledge with additional training
There is no formal exam for becoming a PCQI. That said, whether or not there’s some sort of PCQI test or exercise may vary from provider to provider. While most PCQI trainings simply require course content completion, Registrar Corp’s online course requires that you pass interactive exercises at the end of certain modules with a score of at least 80%. However, if you score below that, you can try again.
Obtaining PCQI certificate of completion is a valuable investment for food safety professionals and food facilities alike. Here are some key reasons why PCQI certification is worth pursuing:
|
Benefits |
Individuals |
Food Facilities |
|
Regulatory Compliance |
Ensures compliance with FSMA |
Ensures compliance with FSMA |
|
Enhanced Food Safety |
Develops expertise in food safety |
Produces safer food products |
|
Competitive Advantage |
Enhances career opportunities |
Provides a competitive edge |
|
Return on Investment |
Increases earning potential |
Reduces regulatory issues |
Investing in PCQI training and certification is a proactive step towards ensuring food safety, regulatory compliance, and the overall success of a food facility. As the next section will explore, PCQI requirements under FSMA make this certification an essential component of a robust food safety management system.
The Food Safety Modernization Act, signed into law in 2011, marked a significant shift in the FDA's approach to food safety. FSMA moved the focus from reacting to foodborne illness outbreaks to preventing them. The law grants the FDA new authorities to regulate the way foods are grown, harvested, and processed, emphasizing prevention, risk-based oversight, and accountability throughout the food supply chain.
The Preventive Controls for Human Food Rule, one of the seven major rules under FSMA, specifically addresses the PCQI requirement. This rule requires covered facilities to have a written food safety plan developed by a PCQI. The food safety plan must include:
The PCQI is not only responsible for developing the food safety plan but also for overseeing its implementation and ensuring ongoing compliance. This includes reviewing records, conducting reanalysis, and making necessary updates to the plan.
Yes, the FDA requires food facilities covered under the Preventive Controls for Human Food Rule to have a PCQI. This requirement applies to domestic and foreign facilities that manufacture, process, pack, or hold human food for consumption in the United States.
There are some exemptions to this rule, such as:
However, even exempt facilities may benefit from having a PCQI to ensure robust food safety practices and to meet customer or third-party certification requirements.
These dates are in the past now, but the compliance deadlines for the Preventive Controls for Human Food Rule were phased in based on business size starting in 2016:
As of September 2018, all covered facilities are required to have a PCQI and a written FSMA-compliant food safety plan in place. Failure to comply with these requirements can result in regulatory action, including warning letters, injunctions, and even criminal prosecution in severe cases.
If you’re striving for third-party certifications like SQF, for example, having a PCQI can greatly facilitate the certification process.
Many third-party certification standards align with FSMA requirements, so having a knowledgeable PCQI to develop and implement a food safety plan as well as respond quickly and accurately during the certification audit will go a long way to getting certified.
Your PCQI should lead the way here. They’re the backbone of your food safety system, especially when it comes to preparing for FDA inspections.
A paper-based FSMS will not set up food businesses for success in the long-run. One of the primary tasks of the PCQI is to regularly review the food safety system to ensure it remains current and effective. This includes food safety plan documents (e.g., HACCP), preventive controls, monitoring processes, and food traceability. See how FoodDocs supports PCQI responsibilities in the two-minute video below:

FoodDocs food safety monitoring system helps PCQIs verify that team members are consistently following all preventive controls outlined in the plan and that they are effective in controlling identified hazards.
This is made easy with built-in corrective actions which make maintaining accurate and complete records easy. During an FDA inspection, the inspector will review your facility's records to ensure compliance with FSMA requirements. The PCQI must verify that all necessary documentation is properly maintained and easily accessible, including:
In addition to having 24/7 access a real-time food safety compliance dashboard, the PCQI can always verify every completed monitoring task to ensure they've been completed to standard.
With FoodDocs' Traceability system, the PCQI can access timestamped traceability logs in seconds, all in one place, without having to look through piles of paper. This digital solution is critical especially in times of recalls, inspections, or audits.
They can use the advanced search function to search for items by product, batch, ingredient, or expiry date. And since all your traceability records are digital, they’re always secured and can be easily pulled up to prove compliance.
Just because the PCQI is leading the way does not mean it’s other team members can sit back and relax. Food safety compliance is a shared responsibility.
That’s why it’s so important for the PCQI to communicate food safety requirements to staff members and ensure that they understand their roles and responsibilities in maintaining a safe food production environment.
This could look like hosting regular (e.g., quarterly or bi-annually) training sessions that educate employees about current food safety practices, preventive controls, and regulatory requirements.
The PCQI can lead these training sessions, providing practical examples and emphasizing the importance of adhering to established procedures. Training should also cover proper documentation practices, as well as how to respond to potential food safety issues or concerns raised during an inspection.
PCQIs can use the Team Management feature in FoodDocs to record food safety trainings, store food safety certifications, and know exactly when each one requires renewal so that everyone is always working in compliance with regulations.
What this ultimately does is empower team members at multiple levels to take ownership of food safety, which creates a strong food safety culture of compliance. Encourage open communication and provide channels for employees to report potential issues or suggest improvements to the food safety system.
Conducting regular mock inspections and audits is an effective way to assess your facility's readiness for an FDA inspection. These simulated inspections help identify potential gaps in your food safety system and provide opportunities for continuous improvement.
Use this FSMA compliance checklist — it’s a great tool for running your internal mock audit.
During a mock inspection, the PCQI or a designated team should walk through the facility, following the same process that an FDA inspector would. For example:
Catching any deficiencies or areas for improvement during the mock inspection will be a lot easier to address before the real FDA inspection.
Running these audits on paper can be tedious and not always reliable to store original records long-term. That's why many food safety teams use the FoodDocs Audits feature.
You'll be able to create a custom internal audit and choose the:
When the audit is ready, team members can complete it from any mobile device — along with any additional file attachments or images — and save it securely in File Storage.
Embracing a culture of continuous improvement will ensure that your facility remains inspection-ready and committed to producing safe, high-quality food products.
A PCQI is essential for ensuring food safety, maintaining FDA compliance, and safeguarding your brand reputation. By developing a comprehensive food safety plan and overseeing its implementation, a PCQI helps identify and control potential hazards, reducing the risk of costly violations and foodborne illness outbreaks.
Investing in PCQI training for your staff is a proactive step towards a more robust food safety culture. With the FDA's increased focus on prevention under FSMA 204 and other rules, having a qualified individual leading the way is no longer optional — it's a necessity.
Check out our food safety compliance software today!
Master restaurant sanitizing with clear steps for kitchens and dining areas. Learn methods, checklists, and procedures to keep your team safe and...
Learn challenges healthcare foodservice teams face today and key food safety practices to protect vulnerable patients. Get a free healthcare leader...
Learn what Standard Operating Procedures (SOPs) are and how to write effective SOPs that ensure consistency, efficiency, and safety in your...