FSMA COMPLIANCE CHECKLIST (FREE DOWNLOAD)




This is how our Digital Food Safety platform saves 20% of your time on daily tasks:
- Get upcoming task notifications
- Add data into the app
- Check the status of tasks in real-time

When food safety was still handled on paper, I typically spent a couple of hours per day getting the papers and going around checking or completing tasks… Now I can sit down and it's just all there in one place. It takes me 5-10 minutes.
Ruth B.
Store Manager
Navigating the complex world of FSMA compliance can feel like a daunting task for food safety professionals.
But with the right guidance and a comprehensive FSMA compliance checklist, you can confidently ensure your facility meets these critical food safety requirements.
In this blog post, we’ll provide you with a detailed FSMA compliance audit checklist and best practices in the food industry to streamline the process.
So, let's dive in and explore how to navigate FSMA compliance standards with confidence and clarity.
Key points covered:
- Domestic and foreign food facilities need to comply with requirements for risk-based preventive controls and the modernized Current Good Manufacturing Practices (cGMPs).
- FSMA offers exemptions and/or modifications for different types of produce, food grains, and certain farms.
- Thorough record-keeping, regular internal audits, and preparation for third-party inspections are key to meeting FSMA compliance.
- Creating a dedicated audit preparation team, developing an audit preparation timeline and checklist, documentation review, and facility readiness are some of the best ways to prepare your team for internal and external FSMA compliance audits.
What are the FSMA compliance requirements and exemptions?
In our article covering FSMA regulations, we shared that the Food Safety Modernization Act aims to protect consumers from potential food safety hazards. For this to be possible — unless otherwise exempt — it mandates all owners, operators, or agents of domestic or foreign food facilities that process or manufacture, package, or hold food for consumption in the U.S. to register under section 415 of the Federal Food, Drug, and Cosmetic Act.
According to the FSMA Final Rule for Preventive Controls for Human Food:
These domestic and foreign facilities “must comply with the requirements for risk-based preventive controls… as well as the modernized Current Good Manufacturing Practices (cGMPs) of this rule.”
The FSMA does highlight exemptions for:
- Produce that is not a raw agricultural commodity
- Produce that is used for personal or on-farm consumption
- Food grains (e.g., including barley, sorghum, oats, rice, rye, wheat, quinoa, buckwheat, oilseeds, dent- or flint-corn, and more)
- Produce commodities that FDA identifies as rarely consumed raw (e.g., asparagus, kidney beans, garden beets, coffee beans, winter squash, sweet potatoes, figs, ginger, hazelnuts, okra, peanuts, peppermint, pumpkins, water chestnuts, and more)
Farms that have an average annual value of $25,000 or less of produce sold during the previous three-year period are also exempt, along with other qualified exemptions and modified requirements for certain farms based on sales and end-user specifications.
3 Best practices for FSMA compliance
These aren’t the only best practices, but they’re ones that are consistent across successful food businesses who are striving to be compliant with FSMA regulations, including the Food Traceability Final Rule.
1. Thorough record-keeping
Maintaining accurate and complete records is essential for FSMA compliance. Food manufacturers must establish a well-organized system to document their food safety practices, including monitoring and traceability, corrective actions, and verification activities (i.e., evidence).
The FSMA Produce Safety Rule specifically mandates farms, for example, to keep records related to agricultural water testing and treatment, biological soil amendments of animal origin, employee training, and cleaning and sanitizing of equipment and tools.
Best practices for record keeping include:
- Using digital tools to streamline data collection and storage
- Establishing clear protocols for record creation, review, and retention
- Training employees on proper documentation procedures
- Regularly reviewing records for completeness and accuracy
2. Regular internal audits
Conducting regular inspections internally will help your business identify FSMA compliance gaps and drive continuous improvement.
Collecting data is just one side of the coin; on the other side is analyzing the data you collect. When you address audit findings regularly, it gives you more opportunities to assess the strengths and weaknesses of your food safety system and reduce risks of non-compliance as well as food safety incidents.
To conduct food safety audits effectively:
- Develop a comprehensive FSMA audit checklist
- Train a dedicated team of internal auditors on audit procedures and techniques
- Schedule audits at regular intervals, covering all aspects of food safety systems
- Document audit findings and develop corrective action plans to address identified issue
- Follow up on corrective measures to ensure their effectiveness and timely implementation
Plus, if you’re trying to create a culture of food safety — be it from the top down or bottom up — regular internal audits will increase the organization's commitment to food safety and keep it top of mind.
3. Objective third-party audits
At the end of the day, internal audits are an essential step in preparation for an accredited third-party certification. They provide businesses with an objective and unbiased independent assessment of their FSMA compliance that can identify blind spots and give your business added credibility in the eyes of consumers.
To successfully navigate third-party audits:
- Choose a reputable audit firm with experience in FSMA compliance
- Conduct a pre-audit assessment to identify and address potential gaps
- Ensure that all necessary documentation is readily available and well-organized
- Communicate openly with auditors and provide them with the support they need
- Use audit results to inform continuous improvement efforts
To reiterate…
Practicing good record-keeping, conducting internal audits, and having a certified FSMA third-party auditor assess your facility will help:
- Enhance credibility with customers and regulators
- Provide objective feedback on the effectiveness of food safety programs
- Support continuous improvement by highlighting areas for further development
FSMA compliance strategies: how to streamline your audit process
Let’s assume that you know which FSMA rule applies to your business and have already designated a trained Preventive Controls Qualified Individual (PCQI) on your team (or hired an external consultant if you’re from a smaller facility).
The PCQI is the best point-person to kick off this process as they’ll have already outlined, managed, and documented your business’s overall food safety plan. Preparing for an FSMA compliance audit internally, but especially with a third-party, can be daunting. But with your PCQI (or equivalent consultant) at the helm, you’ll be successful.
Creating a dedicated audit preparation team
While a single individual might orchestrate the overall plan for FSMA compliance, involving at least one representative from every department is ideal. After all, food safety touches every part of the process.
When you’ve assembled your FSMA audit prep team, give each member specific roles and responsibilities because:
- Complying with FSMA regulations is impossible to do alone
- It’ll give break down silos within the business and empower teammates to own their roles in the food safety plan
“Auditors may seek out signs of responsibility, ownership, and ability to carry out assigned duties according to plan,” says Angela Anandappa, Ph.D. and founding executive director of the nonprofit Alliance for Advanced Sanitation. “This entails not only written standard operating procedures (SOPs) that should be followed as designed, but also the ability to understand and impact change when a problem arises, and to truly understand the ‘why’ behind the ‘what.’”
Developing an audit preparation timeline and checklist
Is your third-party FSMA audit schedule yet? Are you just starting to audit your facility internally? Creating a detailed timeline and checklist for audit preparation helps keep the team organized and on schedule.
The timeline should include items such as:
- Employee training sessions
- Ddocumentation review deadlines
- Facility preparation tasks
Mapping these details out ahead of time will ensure that your team is well-prepared for the internal or external FSMA audit.
And to stay on track, take advantage of your food safety software if you aren’t already and schedule these items in so that your team can receive notifications to complete their audit preparation checklist tasks.
You could use FoodDocs Audits, for example, to create and schedule regularly scheduled internal FSMA audits and assign audit prep tasks using our Monitoring tool so that there’s a timeline and log for your processes.

Documentation review
Thorough documentation is a cornerstone of FSMA compliance. As Dr. Elis Owens, the director of technical services at Birko Corp., says:
“Nearly as important as doing the right things is documenting the who, what, when and where of your food safety processes. If an FDA inspector shows up at your plant, they will ask for documentation and, if it wasn’t documented, it’s as if it never happened. Plus, if you fail to produce the right monitoring, traceability, or audit records, the FDA could shut down production.”
During the audit preparation process, it's crucial to review and update all relevant food safety documents, including HACCP plans, sanitary standard operating procedures (SSOPs), and comprehensive training records. Ensuring that these documents are accurate, up-to-date, and readily accessible is essential for demonstrating compliance during the audit.
Facility readiness
Documentation review goes hand in hand with preparing your facility. During the audit process, they should effectively reflect one another.
Really, if you’re at the stage of getting a third-party FSMA audit done, you should already be doing anything and everything related to keeping your facility safe and sanitary.
And even if it’s an internal FSMA audit you’re preparing for, going through the process of preparing your facility for one will only increase your staff's confidence and consistency in procedures.
It should go without saying, but success in this area will be determined by your business’s level and quality of employee training. Riaz Ahamadeen, Oatly’s Vice President of Quality/Food Safety and Regulatory–North America for Oatly, acknowledges that external training is beneficial. However:
“In-house training allows organizations to include more employees, as external training can be limited by operational needs and costs. When employees learn within the familiar spaces of their workplace, they can better understand and apply the training directly to their tasks. Walking the floor with trainers/professionals and witnessing real-life scenarios can enhance the effectiveness of in-house training.”
Getting your facility ready for its audit should include:
- Conducting a thorough cleaning and sanitation of the entire facility
- Ensuring all equipment is well-maintained and properly calibrated
- Verifying pest control measures are in place and effective
- Reviewing and updating facility layouts and maps
- Ensuring all necessary detailed records and documentation are readily accessible
Key elements of this FSMA compliance audit checklist
In our FSMA audit checklist that will help you meet compliance with industry regulations, we include questions related to the following topics. Together, they address preventive controls, inspection and compliance, imported food safety, as well as response and recovery:
- Scope and Personnel
- Hazard analysis
- Labeling
- Storage
- Staging
- Utensils
- Personnel
- Equipment
- Transportation
- Sanitation
- Raw Materials
- Grounds
- Structure
- Non-contact surfaces
- Plumbing and HVAC
- Non-food chemicals
- Supply Chain
- Intentional Adulteration
- Monitoring
- Validation
- Re-anlaysis
- Recalls
Ready to download your FSMA audit checklist template?
Jump back to the top of the page to fill out, modify, or simply download the template to complete later.
You can also try FoodDocs and create an internal FSMA compliance checklist with the Audits feature!
Once you create your account, simply head over to the Audits feature to create a new one.
You'll be able to create a custom internal audit and choose the:
- Scoring format (e.g., Yes, No, or N/A)
- Exact questions you want to include
- Date(s) that you perform the FSMA audits (along with in-app reminders) Review results in one organized cloud storage and manage anything you need later on.
When the audit is ready, team members can complete it from any mobile device — along with any additional file attachments or images — and save it securely in File Storage.
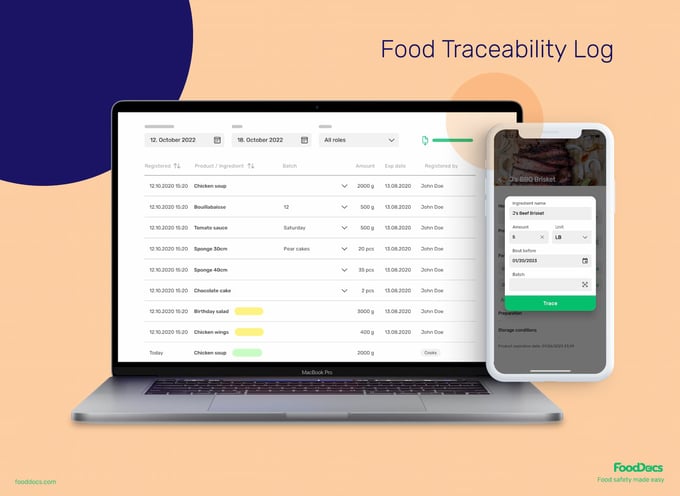
Traceability System for accurate tracking of ingredient and product movement
You can also benefit from our Traceability System by creating traceability logs with just three clicks. Enter food product information, including:
- Product name
- Ingredients
- Batch number/ Production dates
- Target amount
- Expiry date
All essential information needed to track the movement of your ingredients and products and for recalls is conveniently presented in one custom food traceability software.
Managers can attach monitoring tasks to traceability information, such as cooling temperature or dispatch records, to further support record-keeping. They can also attach receiving temperature tasks to ingredients using the monitoring feature and, based on batch numbers, still access relevant traceability data.
In case of a recall or inspection, you can find historical traceability logs in seconds using the advanced search filter. This will give you instant access to information based on entry date, expiry date, product batch, and ingredient batch data.
And if you want to further analyze specific batches of food information, simply download the data as a CSV or XSLS file.
While food safety teams can log traceability information on desktop, logs are much easier to complete with the FoodDocs mobile app — available on both Apple iOS and Android.
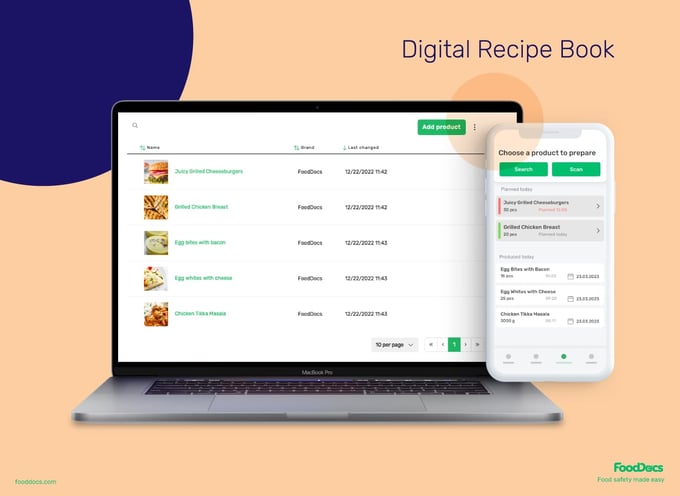
Organize all recipes and allergens with a Digital Recipe Book
Ensure that all of your recipes are organized in cloud storage with the help of our Digital Recipe Book. With this feature, you can log all recipe information, including the following, to our system:
- Recipe name
- Ingredient amounts
- Preparation steps
- Allergen information
- Shelf-life information
- Image of the final product
The information logged into our Digital Recipe Book is also used to automatically calculate how much food you're preparing and expiration dates of products.
Use this smart solution to ensure food handlers have access to proper food preparation and reference for the correct business processes.
Track shelf-life automatically based on information provided in recipes
Once you log individual product or ingredient shelf-life information in our Digital Recipe Book, you can receive intuitive alerts about shelf-life reminders.
With this feature, you can access accurate data for labels and ensure that all food ingredients and products are optimized before spoilage to reduce food waste.
With the help of our comprehensive food traceability software system, you can be confident that all ingredient and product information is well-organized and can be accessed easily in case of future recall events or inspections.
Try FoodDocs today with a 14-day free trial and make your journey to FSMA compliance easier.