Mastering Restaurant Sanitizing: Best Practices for Kitchens and Dining Areas
Master restaurant sanitizing with clear steps for kitchens and dining areas. Learn methods, checklists, and procedures to keep your team safe and...
Discover the 8 practical steps you can start taking today to get compliant with the Food Traceability Final Rule.
In January 2011, President Barack Obama signed the Food Safety Modernization Act (FSMA) into law. Now, the FSMA 204 final rule on food traceability has many businesses needing to adapt and update their food safety management systems.
The FSMA contains many sections but in this article, we'll cover Section 204(d), which outlines additional traceability record-keeping requirements for certain foods.

Food traceability refers to a business's ability to track the movement of food materials throughout the supply chain until they become finished products. In the context of FSMA 204, the traceability rule includes:
FSMA 204, also known as the Food Traceability Rule, is Section 204 of the Food Safety Modernization Act, which requires the FDA to identify foods that require additional recordkeeping to protect public health.
FSMA 204 was written into law for many reasons, one of the primary ones being to more effectively minimize and mitigate food safety hazards (e.g., E. coli and Salmonella). In particular, these additional recordkeeping requirements are intended to identify and remove potentially contaminated food from the market even faster, resulting in fewer foodborne illnesses and worse, deaths.
FSMA 204 applies to businesses that need to keep additional traceability records if they hold, process, pack, or manufacture foods that are specifically on the Food Traceability List. Additional record-keeping requirements include maintaining Key Data Elements and Critical Tracking Events and being able to forward these records to the Food and Drug Administration (FDA), ideally within 24 hours.
The FSMA 204 compliance date was supposed to be Tuesday, January 20, 2026, but will now be sometime in July 2028.
The FDA recognizes that food businesses will need to implement or update their traceability plans. This is something that should not and cannot happen overnight.
As of March 20, 2025:
The FDA announced "its intention to extend the compliance date for the Food Traceability Rule (the “final rule”) by 30 months. The FDA intends to extend the compliance date using appropriate procedures at a later time, including publishing a proposed rule in the Federal Register."
In the past, the FDA website stated:
"We generally note that, while we aren’t initiating routine inspections until 2027, we may do inspections for compliance with the Food Traceability Rule on a for-cause basis, such as during an outbreak investigation, once the compliance date of January 20, 2026, is reached."
This begs the question, since companies have even more time now, will "routine inspections" begin immediately when the new July 2028 compliance date comes into effect? Time will tell.
Before we dive deeper into FSMA 204 compliance, let’s level set about the traceability rule terms and acronyms that we’ll be seeing a lot.

The idea of a designated high-risk foods list goes back to 2014 when, as required by the FSMA law, the FDA requested comments, scientific data, and information help them implement one.
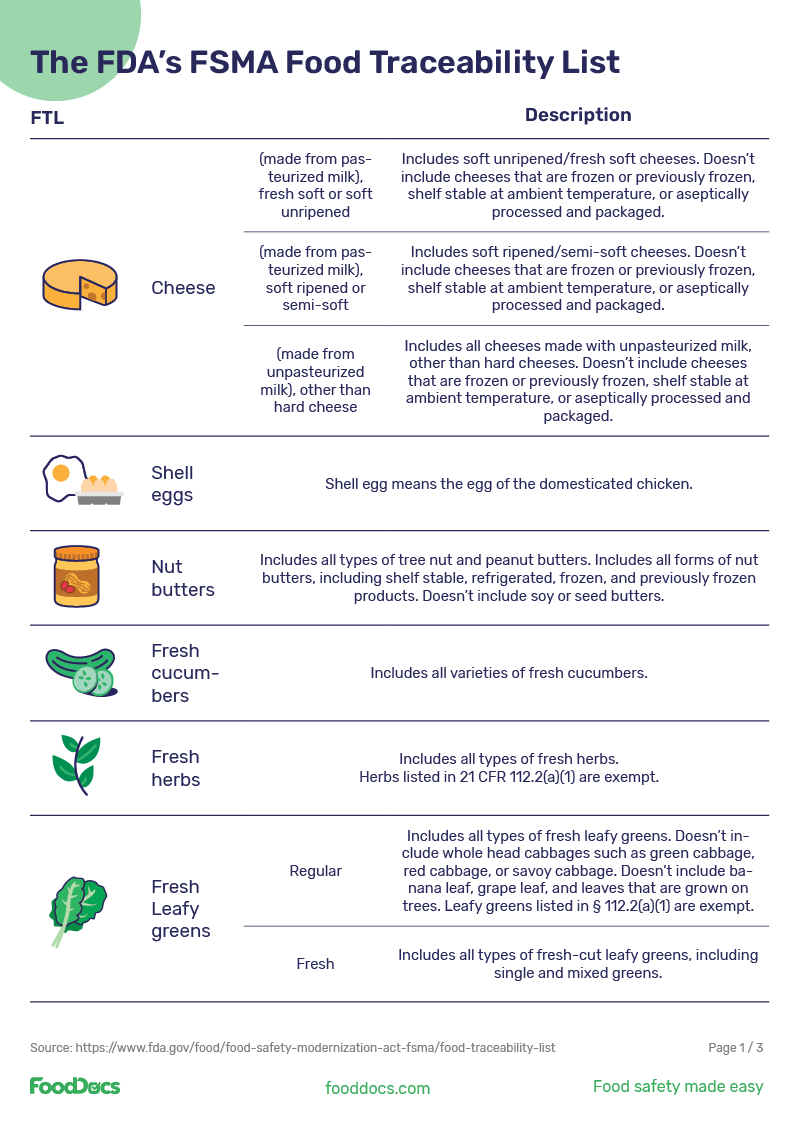
Today, the food traceability list we have is a result of thorough analyses of outbreak investigations and recall data over time. The amount and frequency of outbreaks and recalls helped to determine which foods — from cheeses to popular produce — appear on the food traceability list as outlined in FSMA Section 204.
|
FTL |
Description |
|---|---|
|
Cheese (made from pasteurized milk), fresh soft or soft unripened |
Includes soft unripened/fresh soft cheeses. Doesn't include cheeses that are frozen or previously frozen, shelf stable at ambient temperature, or aseptically processed and packaged. |
|
Cheese (made from pasteurized milk), soft ripened or semi-soft |
Includes soft ripened/semi-soft cheeses. Doesn't include cheeses that are frozen or previously frozen, shelf stable at ambient temperature, or aseptically processed and packaged. |
|
Cheese (made from unpasteurized milk), other than hard cheese |
Includes all cheeses made with unpasteurized milk, other than hard cheeses. Doesn't include cheeses that are frozen or previously frozen, shelf stable at ambient temperature, or aseptically processed and packaged. |
|
Shell eggs |
Shell egg means the egg of the domesticated chicken. |
|
Nut butters |
Includes all types of tree nut and peanut butters. Includes all forms of nut butters, including shelf stable, refrigerated, frozen, and previously frozen products. Doesn't include soy or seed butters. |
|
Fresh cucumbers |
Includes all varieties of fresh cucumbers. |
|
Fresh herbs |
Includes all types of fresh herbs. Herbs listed in 21 CFR 112.2(a)(1) are exempt. |
|
Fresh Leafy greens (fresh) |
Includes all types of fresh leafy greens. Doesn't include whole-head cabbages such as green cabbage, red cabbage, or savoy cabbage. Doesn't include banana leaves, grape leaves, and leaves that are grown on trees. Leafy greens listed in § 112.2(a)(1) are exempt. |
|
Fresh leafy greens |
Includes all types of fresh-cut leafy greens, including single and mixed greens. |
|
Fresh melons |
Includes all types of fresh melons. |
|
Fresh peppers |
Includes all varieties of fresh peppers. |
|
Fresh sprouts |
Includes all varieties of fresh sprouts (irrespective of seed source), including single and mixed sprouts. |
|
Fresh tomatoes |
Includes all varieties of fresh tomatoes. |
|
Fresh fruits from tropical trees |
Includes all types of fresh tropical tree fruit. Doesn't include non-tree fruits, tree nuts, pit fruits, or citrus. |
|
Fresh-cut fruits |
Includes all types of fresh-cut fruits. Fruits listed in § 112.2(a)(1) are exempt. |
|
Fresh-cut vegetables other than leafy greens |
Includes all types of fresh-cut vegetables other than leafy greens. Vegetables listed in § 112.2(a)(1) are exempt. |
|
Finfish, histamine-producing species (fresh, frozen, and previously frozen) |
Includes all histamine-producing species of finfish. |
|
Finfish, species potentially contaminated with ciguatoxin (fresh, frozen, and previously frozen) |
Includes all finfish species potentially contaminated with ciguatoxin. |
|
Finfish, species not associated with histamine or ciguatoxin (fresh, frozen, and previously frozen) |
Includes all species of finfish not associated with histamine or ciguatoxin. Siluriformes fish are not included. |
|
Smoked finfish (refrigerated, frozen, and previously frozen) |
Includes all types of smoked finfish, including cold smoked finfish and hot smoked finfish. |
|
Crustaceans (fresh, frozen, and previously frozen) |
Includes all crustacean species. |
|
Molluscan shellfish, bivalves (fresh, frozen, and previously frozen) |
Includes all species of bivalve mollusks. Doesn't include scallop adductor muscle. Raw bivalve molluscan shellfish that are (1) covered by the requirements of the National Shellfish Sanitation Program; (2) subject to the requirements of 21 CFR part 123, subpart C, and 21 CFR 1240.60; or (3) covered by a final equivalence determination by FDA for raw bivalve molluscan shellfish are exempt. |
|
Refrigerated ready-to-eat deli salads |
Includes all types of refrigerated ready-to-eat deli salads, including ready-to-eat deli salads that are frozen at some point in the supply chain before retail. Doesn't include meat salads. |

Thank you for downloading free template!
Want to get a customizable HACCP template?
Or set up your food safety system in 15 minutes?
In her Food Safety Fridays talk for the ISFQN community, Radojka Barycki, Owner and CEO of RDR Global Partners, shared that the FDA will review the food traceability list every five years. Since the FTL was updated in 2022, the food industry can expect an updated list of foods for inclusion sometime in 2027.
Now that you’re aware of what’s included in the Food Traceability List, you’ve got to know your CTEs and KDEs, which help to form the foundation for more safe and holistic food traceability throughout the entire supply chain.
You can see the original comprehensive list with more in-depth examples of food on the FDA's Food Traceability List page.
Critical Tracking Events are important aspects of a food item's journey that help to decrease likelihood of contamination and reduce risk of foodborne diseases.
There are 7 critical tracking events which include:
![]()
Key Data Elements are different pieces of data that are determined by the specific CTEs which businesses have to keep track of. These details should answer the who (parties involved), what (activities that occurred to the food item), when (date of the event), and where (location).
Different key data elements are required for each critical tracking event. But some common KDEs include:
For exact details and wording of what key data elements are needed for each critical tracking event, reference the FDA USDA's CTE and KDE document. (Yes, that was a lot of acronyms...)
Traceability Lot Codes or TLCs are unique identifiers (usually alphanumeric) assigned to items on the food traceability list to increase supply chain transparency You must assign a TLC to a food on the Food Traceability List (FTL) when you:

|
Attribute |
Details |
|
1: Traceability lot code |
Consensus on using GTIN alongside a Lot Code |
|
2: Quantity & unit of measure |
Examples include 1 case, 10 reusable containers, 100 storage tanks, 1000 pounds |
|
3: Product description |
Details such as product name, brand, type of commodity, variety, and packaging specifics including size or style, and types of seafood |
|
4: Location description for receiver |
Details like business name, contact number, full address including city, state, zip, and country |
|
5: Location description for source |
Includes business name, contact information, and complete address with city, state, zip, and country |
|
6: Date |
Records the date of when the food was either shipped or received |
|
7: Traceability Lot Code Source (TLC Source) Traceability Lot Code Source Reference (TLC Source Reference) |
The location where a food item was given its traceability lot code, noting the company's address, city, state, zip, and contact. An alternative method to detail the location for the TLC Source, potentially including a web address, FFRN, DUNS, or GLN, with accessibility mandated by the FDA. |
|
8: Reference documents |
Includes any business document or record that holds key data elements for an event, such as BOL, PO, ASN, work orders, invoices, receipts, etc. |
|
§ 1.1340: Shippers must provide (via electronic, paper, or another written method) all details specified in this section to the next direct recipient (excluding transporters) of any shipped traceability lot. |
|

A number of businesses are exempt from food traceability with either full exemptions, partial exemptions, or full and partial exemptions. You can find the exact answer to “What foods and persons are exempt from this subpart?” in Section 1.1305 in the Code of Federal Regulations.
Here’s a high-level overview of the business types that fall under these exemption categories:
It’s important to note that exemptions aren't always clear-cut. Sometimes, a company might not need to keep certain records for some types of food but will need to keep them for others.
Also, a company might be exempt from some rules at one stage of moving food from place to place, but later stages could still require following those rules. This shows how some foods that usually don't need detailed records might still need them when they reach someone else down the line.
Want to see whether or not your business is exempt from FSMA 204 regulations, use the FDA’s Exemptions to the Food Traceability Rule interactive flow chart.
(If you’d like, try the exemption tool above to clarify if you will have to seek FSMA 204 compliance before jumping into the steps below.)
Below, we’ll get into the practical steps you can start taking to ensure your food business is ready for FSMA 204 compliance in time for January 20, 2026:
To ensure your business aligns with FSMA 204 regulations, start by forming a compliance team. This group should be composed of key personnel who understand the complexities of your food operations.
Including a mix of roles, such as operations managers, quality assurance specialists, and IT staff, is beneficial to cover all aspects of the compliance process. Importantly, the team should be led by a Preventive Controls Qualified Individual (PCQI), who brings essential knowledge of FSMA requirements.
When assembling your team, consider the scope of your operations and the specific needs related to FSMA 204 compliance, such as traceability and preventive controls, ensuring each member is clear on their roles and responsibilities. In a typical food business, for example, an FSMA 204 compliance team could look like this:
|
Role |
Responsibilities |
|
Operations Manager |
Manages the integration of traceability systems and ensures all processes comply with FSMA 204 requirements. |
|
Quality Assurance Specialist |
Develops and maintains preventive controls, conducts hazard analyses, and ensures the food safety plan meets regulatory standards. |
|
IT Staff |
Implements and maintains technology solutions to support traceability and record-keeping required by FSMA 204. |
|
PCQI (Preventive Controls Qualified Individual) |
Leads the development, implementation, and review of the food safety plan; trains team members on compliance-related tasks. |
This structured approach will not only facilitate smoother implementation but also enhance ongoing compliance efforts. If you’ve already got a PCQI to generally maintain FSMA compliance, you’ll be off to a good start.
Knowledge is power and knowing what the food traceability rule expects of you will make the compliance journey much easier. Start by reviewing the official final rule and lean on resources like the one you’re reading now. Build a comprehensive resource list that includes eBooks, webinars, and presentations from the FDA and other experts to deepen your understanding.
Here’s a starter list:
Also, refer back to the glossary section earlier in this article to familiarize yourself with key terms like FTL, CTE, KDE, and TLC, ensuring that all team members have a solid foundation in the terminology and requirements of FSMA 204.
Understanding the traceability rule's impact on your specific business operations is crucial. Take these steps to help determine the scope of FSMA 204 compliance:
Essentially, assess your existing food traceability program to ensure it meets the transparency and safety requirements throughout your supply chain.
Start by pinpointing your company's role in the supply chain to understand your specific responsibilities under FSMA 204. Do you hold, process, pack, or manufacture foods? Is it a combination?
Examine your current systems for tracking and managing traceability data to see how well they align with FSMA requirements. Check how you currently receive, or plan to receive, traceability data from your suppliers.
to help with FSMA 204 compliance
Engage in discussions with your customers, trading partners, and technology providers to gather insights on their FSMA 204 compliance strategies. In addition to creating an FSMA compliance statement of your own, request to see your supplier’s statement to make sure their food safety compliance goals align with your company’s.
Evaluate whether your trading partners are prepared for FSMA compliance, ensuring that all links in your supply chain are ready to meet these regulatory requirements. You can start with your approved suppliers list and based on their FSMA compliance (or lack thereof), you can decide whether you’ll need to source new ones.
This holistic approach will help you identify any gaps in your current system and facilitate a smoother transition to full FSMA 204 compliance.
Compare your current systems to FSMA 204 requirements to uncover any discrepancies. Common gaps might include inadequate data capture capabilities or insufficient traceability measures for items on the Food Traceability List.
Review the impact of these gaps on your systems, hardware, and operational procedures.
You should also assess potential risks in your traceability system, such as the vulnerabilities of a paper-based system versus the benefits of adopting food traceability software. Searching through piles of paper for specific food safety monitoring records when the FDA gives you just 24 hours to provide the recall data becomes a lot more stressful and difficult. With digital traceability logs, you can share the information with the FDA in seconds.
To effectively implement your FSMA 204 compliance plan:
For effective traceability management, assign role-based traceability tasks using your chosen system — paper-based or digital. Here's a list of typical traceability monitoring tasks in a food business:
Managing these tasks with a paper system can be challenging due to the manual effort required, the potential for human error, and difficulties in quickly accessing specific data during audits or inspections.
Transitioning to a digital traceability system offers significant benefits, such as real-time data updates, improved accuracy, and easier access to traceability records, enhancing overall food safety compliance and operational efficiency.
Regularly monitor and review your food traceability program and FSMA 204 compliance, establishing a review cadence that aligns with your business operations.
Incorporate training sessions and mock audits as part of this ongoing process to keep your team sharp and prepared. You can use the FSMA compliance checklist and place a particular focus on the final Recall section.
Be prepared to re-assess your compliance strategies, particularly when significant operational changes occur or when new regulations are introduced, such as the FDA's updates to the Food Traceability List every five years. This approach ensures that your compliance measures evolve and improve continuously, staying effective and relevant.
January 2026 seems far away, but in the context of food safety and the change management inevitably involved in implementing a plan and system that works for your team, it will approach faster than you think.
Getting buy-in at every level of the business, sourcing new or better compliant suppliers, and making other necessary changes in your organization can take several months or even over a year.
And why wouldn't you? If you're a business that's directly impacted by FSMA Rule 204, getting compliant will help to:

Look at that, those four bullet points create an apt acronym: PREP. And on that note...
Regarding having what you need, many businesses choose to digitize their food safety system. Leveraging the right food safety software will help your team:
Regardless of what food traceability software you choose, make sure it's able to support the specifics outlined earlier in FSMA Section 204's key features: critical tracking events and key data elements, traceability lot codes, traceability plan, as well as additional requirements.
Would you like to make traceability logging and recall management easier? FoodDocs’ software is built so you can log your traceability within seconds, saving time on repetitive activities and making the whole traceability process easy to trace in case of a recall.

Using FoodDocs' smart Food Safety Management System equipped with an all-in-one effective Traceability System fulfills your production and traceability needs.
In addition to the most critical and primary features of a food safety management platform, our smart software features a Traceability System with the following benefits:
You can create traceability logs with just three clicks. Enter all product information, including:
All essential information needed to track the movement of your ingredients and products and for recalls is conveniently presented in one custom food traceability software.
Managers can attach monitoring tasks to traceability information, such as cooling temperature or dispatch records, to further support record-keeping. They can also attach receiving temperature tasks to ingredients using the monitoring feature and, based on batch numbers, still access relevant traceability data.
In case of a recall or inspection, you can find historical traceability logs in seconds using the advanced search filter. This will give you instant access to information based on entry date, expiry date, product batch, and ingredient batch data. And if you want to further analyze specific batches of food information, simply download the data as a CSV or XSLS file.
While food safety teams can log traceability information on desktop, logs are much easier to complete with the FoodDocs mobile app — available on both Apple iOS and Android.
Ensure that all of your recipes are organized in cloud storage with the help of our Digital Recipe Book. With this feature, you can log all recipe information, including the following, to our system:
The information logged into our Digital Recipe Book is also used to automatically calculate how much food you're preparing and the expiration dates of products.
Use this smart solution to ensure food handlers have access to proper food preparation and reference for the correct business processes.
Once you log individual product or ingredient shelf-life information in our Digital Recipe Book, you can receive intuitive alerts about shelf-life reminders.
With this feature, you can access accurate data for labels and ensure that all food ingredients and products are optimized before spoilage to reduce food waste.
With the help of our comprehensive food traceability software system, you can be confident that all ingredient and product information is well-organized and can be accessed easily in case of future recall events or inspections.
Whether you're holding, manufacturing, processing or packing items on the Food Traceability List, you can gain a competitive advantage by using food traceability software. Give FoodDocs' a try with a 14-day free trial and take your next step toward FSMA 204 compliance.
A group of U.S. politicians recently introduced legislation called the Food Traceability Enhancement Act. It proposes implementation changes which aim to “strengthen compliance” with the Food Traceability Final Rule.
If the bill ends up passing, it would lessen the TLC and record-keeping requirements for restaurants, food retailers, and warehouse facilities. Specifically, these types of businesses would no longer be required to maintain TLC data nor send the data to supply chain partners or to the Secretary of the U.S. Department of Health and Human Services (the Secretary).
Another change, in particular, includes delaying the compliance date to at least two years after the completion of the pilot projects, of which the bill proposes nine or more that the Secretary would have to conduct.
The proposed pilot projects would seek to gauge the effectiveness of foodborne illness investigations without available traceability lot code data, as well as evaluate low-cost food traceability technologies.
For more information about the Food Traceability Enhancement Act, here's the official proposed bill.
The FDA outlines four key features of FSMA 204:
CTEs and KDEs help to form the foundation for more safe and holistic food traceability throughout the entire supply chain.
In the context of the FDA's Food Traceability List (which we include in full below), Critical Tracking Events are important aspects of a food item's journey that help to decrease likelihood of contamination and reduce risk of foodborne diseases.
Critical tracking events include:
Key Data Elements are different pieces of data that are determined by the specific CTEs which businesses have to keep track of.
Different key data elements are required for each critical tracking event. But some common KDEs include:
For exact details and wording of what key data elements are needed for each critical tracking event, reference the FDA USDA's CTE and KDE document. (Yes, that was a lot of acronyms...)
Historically, the FDA has defined "traceability" as the point at which you receive raw materials to the time you process and ship a food product. But in light of continued recalls and a need for faster identification when it comes to the source of contamination, the FDA's addition to the FSMA makes it clear that the traditional one-step-forward, one-step-backward record-keeping rule is insufficient for accomplishing true food traceability.
That's why, in order to go more than one step forward or backward, the FDA is placing greater emphasis on stricter documentation and record-keeping of those critical tracking events.
To increase supply chain transparency, a TLC, usually alphanumeric, is a unique identifier assigned to items on the food traceability list. You must assign a TLC to a food on the Food Traceability List (FTL) when you:

As a business that's subject to FSMA 204 requirements, your traceability plan must include details covering:
FSMA 204 also requires that businesses must:
GS1 plays a crucial role in FSMA 204 compliance by offering standardized tools and guidelines that align with the FSMA 204 Final Rule's requirements. Here's an in-depth look at how GS1 aids in compliance:
Formation of the GS1 US FSMA 204 workgroup: GS1 US created a team of industry experts dedicated to formulating guidelines for applying GS1 Standards to fulfill FSMA 204 requirements. This joint initiative highlights the importance of collaboration in improving traceability and compliance.
Guidance document: The GS1 US Application of the GS1 System of Standards to Support FSMA 204 is an essential resource. It details best practices for implementing GS1 Standards to improve the scalability, precision, and interoperability of traceability programs, thereby directly supporting FSMA 204 compliance.
Mapping of standards to FSMA 204 requirements: GS1 US has effectively demonstrated how its standards align with the Key Data Elements (KDEs) and Critical Tracking Events (CTEs) mandated by FSMA 204. This involves utilizing Global Location Numbers (GLN), the Global Data Synchronization Network (GDSN), the Global Data Model (GDM), Electronic Data Interchange (EDI) with Advanced Ship Notice (ASN), and Electronic Product Code Information Services (EPCIS). Each of these elements is crucial for supplying the necessary data and support to monitor a product’s lifecycle within the supply chain.
Support for emerging technologies: GS1 Standards promote the creation and adoption of advanced traceability systems by improving data sharing efficiency and minimizing data entry errors across various platforms. For instance, EPCIS enhances the detailing of CTEs from harvest to sale, ensuring a unified and precise account of supply chain events.
Continued collaboration with the FDA: GS1 US maintains a close partnership with the FDA to guarantee that communication and educational materials about GS1 Standards are accessible and understandable, which is essential for achieving industry-wide compliance with FSMA 204.
In an effort to improve food supply chain visibility and transparency, FSMA 204 mandates the what, i.e., improved record-keeping and reporting of traceability lot codes. But what FSMA 204 does not do is mandate the how. Specifically, it doesn't require food businesses to fulfill these new requirements in a particular way (i.e., paper records vs electronic records).
However, given that the FDA requires you to submit traceability data with 24 hours (unless you establish another agreed upon timeline), paper records could pose more challenges that prevent you from doing it in time, such as:
Frank Yiannis, former FDA Deputy Commissioner, also notes monitoring records that the Traceability Rule doesn't require, including:
As of May 2024, a group of U.S. politicians recently introduced legislation called the Food Traceability Enhancement Act. It proposes implementation changes which aim to “strengthen compliance” with the Food Traceability Final Rule.
If the bill ends up passing, it would lessen the TLC and record-keeping requirements for restaurants, food retailers, and warehouse facilities. Specifically, these types of businesses would no longer be required to maintain TLC data nor send the data to supply chain partners or to the Secretary of the U.S. Department of Health and Human Services (the Secretary).
Another change, in particular, includes delaying the compliance date to at least two years after the completion of the pilot projects, of which the bill proposes nine or more that the Secretary would have to conduct.
The proposed pilot projects would seek to gauge the effectiveness of foodborne illness investigations without available traceability lot code data, as well as evaluate low-cost food traceability technologies.
For more information about the Food Traceability Enhancement Act, here's the official proposed bill.
Update: On March 20, 2025, the FDA announced that it plans to push the FSMA 204 compliance date back by over two years, to around July 2028.
The sentiment among food safety workers isn't the most positive. There are many people who are unsure of how to properly move forward, and not for lack of trying. The IFSQN forum saw a recent topic titled "Is anyone else struggling with the FSMA204 Final Rule regulations?"
People who responded shared legitimate concerns, such as:
This is not a one-dimensional issue. It involves management, the state of food safety technology, those who are writing and enforcing FSMA 204, and more.
These concerns are exactly why, if at this moment, regardless of whether or not the FSMA 204 compliance date gets delayed, you know that you're food traceability plan is non-existent or not where it needs to be, our message is this: do not wait.
In the Episode 97 of Don't Eat Poop! A Food Safety Podcast, hosts Matt and Francine discussed the challenges of the FSMA 204 rollout with Andrew Kennedy.
Kennedy used to work in the FDA and was one of the key people involved in writing the FDA's Traceability Rule. In comparing the traceability rule to GFSI's initial introduction, he said:
No one complains about it anymore because at first when GFSI was rolled out, it was a big deal and people complained and it was hard, but now it's just how people do business...
So I think that's where traceability will be in five, six years, where it will just be how people ship product. And if you don't do traceability, you'll be an outlier.
So I think the transition happens quickly. There's like a tipping point where you go from no one does traceability to everyone does traceability. And then if you want access to markets, you have to do it.
Master restaurant sanitizing with clear steps for kitchens and dining areas. Learn methods, checklists, and procedures to keep your team safe and...
Learn challenges healthcare foodservice teams face today and key food safety practices to protect vulnerable patients. Get a free healthcare leader...
Learn what Standard Operating Procedures (SOPs) are and how to write effective SOPs that ensure consistency, efficiency, and safety in your...