ISO 22000 INTERNAL AUDIT CHECKLIST FREE DOWNLOAD




This is how our Digital Food Safety platform saves 20% of your time on daily tasks:
- Get upcoming task notifications
- Add data into the app
- Check the status of tasks in real-time

When food safety was still handled on paper, I typically spent a couple of hours per day getting the papers and going around checking or completing tasks… Now I can sit down and it's just all there in one place. It takes me 5-10 minutes.
Ruth B.
Store Manager
The food safety compliance equation is simple: audit preparedness goes up, audit anxiety goes down.
While conducting an ISO 22000 internal audit is no small feat, there's much your food safety team can do to ace it while increasing the overall business's food safety culture.
One part of achieving that success is using the right ISO 22000 internal audit checklist along with best practices that confidently ensure your organization's compliance and continual improvement.
Whether you're a seasoned quality manager or have entered a new role that's placing food safety audits on your work plate, this article will equip you with the knowledge and tools to excel in your next ISO 22000 internal audit.
Let's dive in and explore how you can further elevate your food safety management system's compliance!
Key points covered:
- ISO 22000 is a food safety management standard that helps businesses in the food chain follow and continuously improve upon their food safety.
- Having a plan for your internal ISO 22000 audit helps food businesses systematically document their food safety management system.
- Some of the key elements included in this ISO 22000 internal audit checklist are questions about Process Flow Diagram, Hazard Analysis & Preventive Measures, Critical Control Points, Critical Limits, Monitoring Procedures, Corrective Actions, Verification Procedures, Documentation & Record Keeping.
- Best practices for an effective ISO 22000 internal audit are i) fostering a food safety culture of continuous improvement, ii) adopting a risk-based approach, iii) regularly reviewing and updating the FSMS.
- FoodDocs Food Safety Management software can help with all six steps to conduct a thorough ISO 22000 internal audit.
What is ISO 22000? (A quick refresher)
The ISO 22000 is an international standard that's recognized as a set of Food Safety Management System (FSMS) requirements which food producers of all sizes can follow to continuously improve their food safety and provide evidence of their ability to control food safety hazards. ISO 22000 is one of many ISO-based standards that the International Organization for Standardization developed.
What’s the purpose of an ISO 22000 internal audit plan?
The purpose of having a plan for your internal ISO 22000 audit — i.e., outline the objectives, scope, and methodology — is to be able to systematically document your business’s food safety management system against the ISO 22000 standard’s requirements.
Does your existing FSMS conform to ISO standards? Do you know what key processes and areas impact food safety and who’s involved in each one, such as: raw material procurement and supplier management, food production and processing, storage and distribution, cleaning and sanitation, as well as employee training and personal hygiene practices? Is there a timeline for when you’d like to conduct your internal ISO 22000 audits (e.g., every three, six, or twelve months), or prepare for one with external auditors?
Your ISO 22000 internal audit plan will help solidify answers to these questions.
3 Key benefits of using an ISO 22000 internal audit checklist template
Using ISO 22000 audit checklist templates — including this one — offers several advantages for organizations conducting internal audits:
- Ensure a consistent and structured approach to your audits: this reduces the risk of overlooking critical areas or requirements and helps maintain the integrity of your food safety management system and facilitates continuous improvement.
- Save valuable time and resources: rely on a standardized template that’s been carefully crafted (like the one at the top of this page) to cover all relevant aspects of the ISO 22000 standard. This allows your audit team to focus their efforts on conducting the audit itself, rather than spending excessive time on administrative tasks.
- Customize the audit template to fit your organization’s specific needs: Use the ISO 22000 internal audit checklist as is, or do things like modify text and even remove rows entirely if they don’t apply to your business context. This built-in flexibility helps keep your internal audits focused only on your unique food safety challenges and objectives.
Key elements of our ISO 22000 audit checklist template
Using a well-designed ISO 22000 audit checklist should cover all relevant clauses to ensure that the time you spend internally auditing your facility against ISO standards is worthwhile. The template should also include a section for comments and/or evidence to record observations, nonconformities, and corrective actions.
The more thorough you are during the internal auditing process, the less anxious you’ll be when it’s time for the third-party audit.
Our ISO 22000 internal audit checklist includes questions under the following categories:
- Process Flow Diagram
- Hazards Analysis & Preventive Measures
- Critical Control Points
- Critical Limits
- Monitoring Procedures
- Corrective Actions
- Verification Procedures
- Documentation & Record Keeping
- Staff Training
- Recall Plan & Management
- Complaint Management
- Sanitation Control
- Internal HACCP Audit
- Management Responsibility
- Product Specification
The ISO 22000 internal audit checklist above gives you a solid foundation on which to begin. It includes questions organized by topic, a Yes/No/Not Applicable column, and a column to include objective evidence of conformity.
If you want more details and best practices, keep scrolling!
6 Steps to conducting a thorough ISO 22000 internal audit
(And how FoodDocs can support those steps!)
1. Review documentation and records
A thorough review of your food safety management system documentation is crucial to the success of your internal audit. Begin by verifying the existence and effectiveness of all relevant documents, including:
- Food safety policy and objectives
- Hazard analysis and critical control point (HACCP) plan
- Prerequisite programs (PRPs)
- Standard operating procedures (SOPs)
- Work instructions
Ensure that these documents are up-to-date, easily accessible, and well-understood by all employees. Next, check the accuracy and completeness of records related to food safety, such as:
- Monitoring records for critical control points (CCPs)
- Cleaning and sanitation logs
- Training records
- Supplier evaluations and approvals
Pay close attention to any discrepancies or gaps in the documentation and records, as these may indicate potential nonconformities or areas for improvement.
How FoodDocs supports food safety file storage
FoodDocs stores all of your food business's compliance documents in one place with safe and secure cloud storage. You can access these anytime, anywhere so that during external audits, you can quickly and easily pull up whatever document they're asking about.
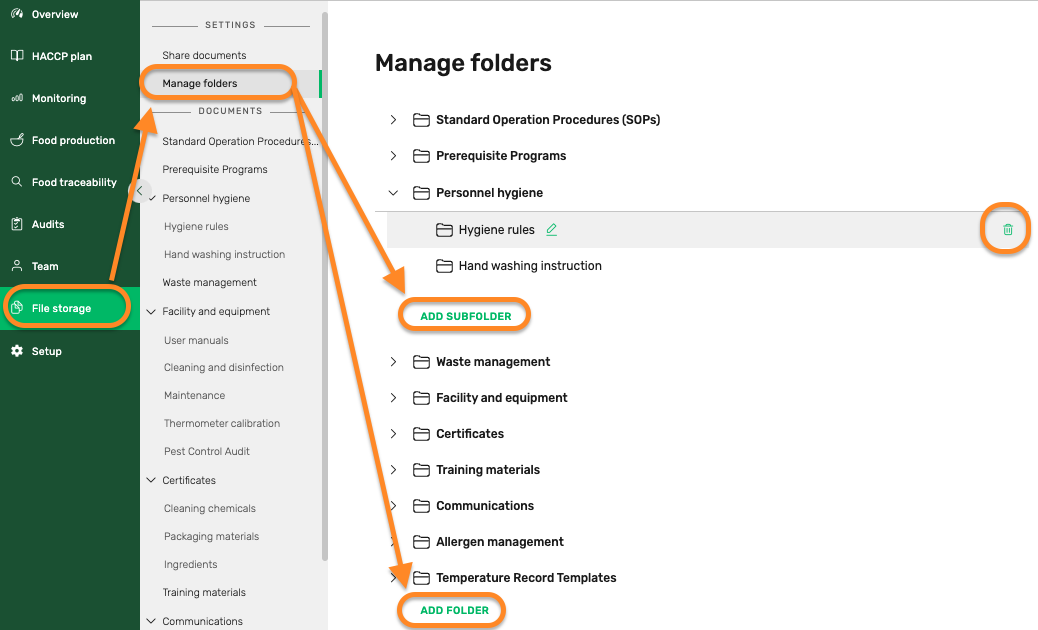
Preview of the cloud file storage feature in the FoodDocs' food safety software where you organize and store every type of file you need for you butchery to stay compliant.
2. Assess operational processes and procedures
With the documentation processes review complete, it's time to evaluate the implementation of your food safety processes and procedures on the ground. Start by observing and assessing the effectiveness of your prerequisite programs, which form the foundation of your FSMS. These may include:
- Good manufacturing practices (GMPs)
- Cleaning and sanitation standard operating procedures
- Pest control measures
- Personnel hygiene practices
Observe employees as they carry out their daily tasks, and conduct interviews to gauge their understanding of and adherence to food hygiene and safety procedures. Look for any deviations from established protocols or potential food safety hazards, such as:
- Improper food handling or storage
- Inadequate cleaning or sanitation
- Lack of personal protective equipment
- Malfunctioning equipment or infrastructure
Document any nonconformities or areas for improvement identified during this step, as they will need to be addressed through corrective actions.
How FoodDocs increases consistent and correct food safety task completion
Make food safety training easier and more efficient with the help of our software's step-by-step instruction tools. Each automatically generated monitoring task comes with detailed instructions on how to perform and monitor the task.
You can use this feature to train food handlers and remind them of the proper execution of tasks according to business's requirements for food safety. In addition, you can use these instructions to train new food workers and save time from conducting food safety training repeatedly.
And if you have any custom monitoring tasks, you can upload your own versions of the instructions in text as well as photo or video format.

Step-by-step instructions from FoodDocs software.
3. Verify corrective and preventive actions
An effective internal audit not only identifies nonconformities but also verifies the effectiveness of corrective and preventive actions taken to address them. Begin by reviewing the status and effectiveness of corrective actions implemented for any nonconformities identified during previous audits or inspections. This may involve:
- Reviewing corrective action reports and related documentation
- Observing the implementation of corrective measures in practice
- Interviewing employees to assess their understanding and adherence to the corrective actions
Next, evaluate the implementation and effectiveness of preventive measures designed to mitigate food safety risks. These may include:
- Hazard analysis and risk assessment
- Employee food safety awareness training and competency programs
- Equipment maintenance and calibration schedules
- Supplier evaluation and approval processes
Ensure that these preventive measures are well-documented, effectively implemented, and regularly reviewed for continuous improvement.
How FoodDocs automates corrective and preventive actions in the food supply chain
Detect risk early to help team members maintain conformity to requirements, reduce product waste, and exceed customer requirements and satisfaction.
 FoodDocs links risk detection with corrective quality control measures. When our food safety software recognizes a risk, you'll get a suggested corrective action notification and be able to correct it and mitigate the risk.
FoodDocs links risk detection with corrective quality control measures. When our food safety software recognizes a risk, you'll get a suggested corrective action notification and be able to correct it and mitigate the risk.
4. Conduct a traceability exercise
A robust food traceability system is essential for quickly identifying and resolving food safety issues, as well as facilitating product recall procedures when necessary. During your internal audit, conduct a traceability exercise to test the effectiveness of your system. This may involve:
- Selecting a finished product and tracing its ingredients back to their sources
- Tracking a raw material forward through the production process to the final product
- Verifying the accuracy and completeness of traceability records, such as batch numbers and lot codes
Document findings from your traceability exercise, including any gaps or weaknesses identified, and develop corrective follow-up actions to address them.
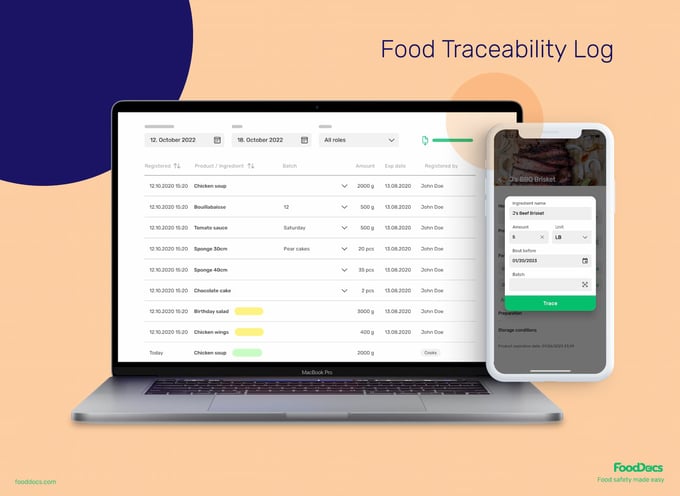
How FoodDocs simplifies food traceability
The Food Traceability System lets you create traceability logs with just three clicks. Enter all product information, including: product name, ingredients, batch numbers, production dates, target amount, and shelf-life expiry date.
All essential information needed to track the movement of your ingredients and products and for recalls is conveniently presented in one custom food traceability software.
Food Safety and Quality Managers can attach monitoring tasks to traceability information, such as cooling temperature or dispatch records, to further support record-keeping. They can also attach receiving temperature tasks to ingredients using the monitoring feature and, based on batch numbers, still access relevant traceability data.
In case of a recall or inspection, you can find historical traceability logs in seconds using the advanced search filter. This will give you instant access to information based on entry date, expiry date, product batch, and ingredient batch data. And if you want to further analyze specific batches of food information, simply download the data as a CSV or XSLS file.
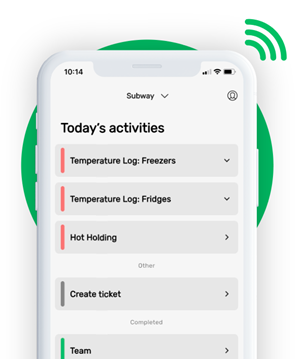
 While food safety teams can log traceability information on desktop, logs are much easier to complete with the FoodDocs mobile app — available on both Apple iOS and Android.
While food safety teams can log traceability information on desktop, logs are much easier to complete with the FoodDocs mobile app — available on both Apple iOS and Android.
5. Review food safety training and competency
Well-trained and competent staff are critical to the success of your food safety system. During your internal audit, review the effectiveness of your food safety training and competency programs. This may include:
- Reviewing training records and materials
- Observing employees to assess their food safety knowledge and practices
- Conducting interviews or assessments to gauge employee understanding and competency
Ensure that your training programs are up-to-date, relevant to your operations, and effectively delivered to all employees. Identify any gaps in employee knowledge or competency and develop corrective actions, such as additional training or coaching, to address them.
How FoodDocs makes team training management easy
As you set up your food business’s HACCP system, you’ll be able to add team members and organize them into specific types of roles. For example: Management, Cooks, Cleaning, Maintenance, Delivery, Customer Service, and more.
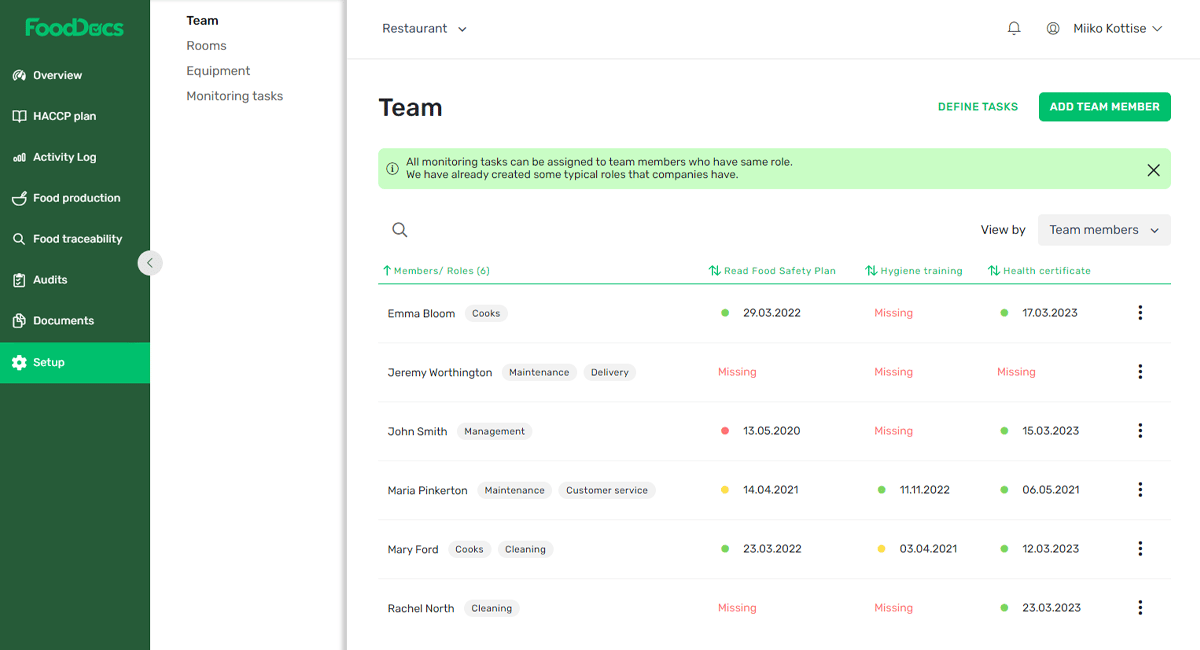
Preview of the Setup page in FoodDocs where you can add team members, define role-based tasks, and have an overview of each person's food safety status.
6. Summarize findings and develop an action plan
With the internal audit complete, it's time to summarize your findings and develop an action plan for continuous improvement. Begin by compiling all nonconformities, observations, and areas for improvement identified during the audit. Classify each finding based on its severity and potential impact on food safety, such as:
- Critical nonconformities that pose an immediate threat to food safety
- Major nonconformities that may lead to food safety issues if not addressed
- Minor nonconformities or observations that do not directly impact food safety but require attention
For each finding, develop a corrective action plan that includes:
- A clear description of the nonconformity or area for improvement
- The root cause analysis and proposed corrective actions
- Responsibilities and timelines for implementation
- Verification measures to ensure the effectiveness of the corrective actions
Present your findings and action plan to senior management for review and approval, and ensure that all corrective actions are implemented in a timely and effective manner.
Following these steps for your ISO 22000 internal audit will help to ensure that your food safety management system is effectively implemented, maintained, and continually improved. Regular internal audits not only help you maintain compliance with the standard but also demonstrate your commitment to producing high-quality, safe products for your customers.
How FoodDocs Audits get you ISO 22000-ready
Use the Audits feature to create a new audit, choosing the scoring format (e.g., Yes, No, or N/A), exact questions you want to include, and date(s) that you perform the FSMA audits (along with in-app reminders).
When the audit is ready, team members can complete it from any mobile device — along with any additional file attachments or images — and save it securely in File Storage.
How to prepare for a smooth internal ISO 22000 audit
Thorough preparation is key to a successful ISO 22000 internal audit in the food industry. Here are some tips that will help you get ready:
Establish an audit schedule and team
Develop a well-defined audit schedule that covers all relevant areas of the FSMS within a reasonable timeframe. Consider factors such as the complexity of processes, previous audit results, and available resources when determining the audit frequency and duration.
Assemble a competent and impartial audit team. Auditors should have a thorough understanding of ISO 22000 requirements, food safety principles, and auditing techniques. Consider providing additional training or certification for internal auditors to enhance their skills and credibility.
Engage and train employees
Any food safety team leader will know that engaging and training employees is crucial for a successful ISO 22000 internal audit.
Communicate the importance and purpose of the audit to all employees, emphasizing how it contributes to the organization's food safety goals and continuous improvement. Provide comprehensive training on ISO 22000 regulatory requirements and internal audit procedures, ensuring that everyone understands their roles and responsibilities.
Employees should feel comfortable raising concerns, suggesting improvements, and sharing their insights. So encourage them to offer you feedback. This collaborative approach fosters a culture of transparency and ownership, leading to more effective audits and better overall food safety performance.
Conduct a pre-audit assessment
Before the actual internal audit, conduct a thorough pre-audit assessment. This involves performing a gap analysis to identify potential weaknesses in the food safety management system. Review documentation, observe processes, and interview employees to uncover any areas that may not fully comply with ISO 22000 requirements.
Address any identified gaps or nonconformities before the internal audit. This proactive approach allows you to rectify issues, update documentation, and implement necessary improvements, reducing the likelihood of major findings during the actual audit. Ensure that all necessary documentation and records are readily available, organized, and up-to-date.
Gap Analysis Techniques
When conducting a gap analysis, consider using the following techniques:
- Process mapping: Visually represent your food safety processes to identify potential gaps or inefficiencies.
- Checklist-based assessments: Use ISO 22000 requirements as a checklist to systematically evaluate compliance.
- Employee interviews: Engage with employees to gather insights and identify areas for improvement.
Review and update documentation
Comprehensive and accurate documentation is essential for a successful ISO 22000 internal audit. Review and update all relevant documents, many of which we mentioned above.
Ensure that documents are current, properly controlled, and easily accessible to auditors and employees. Regularly review and update documentation to reflect changes in processes, regulations, or best practices.
By following these ISO 22000 audit preparation tips, organizations can ensure a smooth and effective internal audit process. Thorough preparation, employee engagement, and a focus on continuous improvement lay the foundation for a robust and resilient food safety management system.
Best practices for effective ISO 22000 internal audits
Adopt a risk-based approach
Prioritizing audit activities based on identified food safety risks is a key best practice in ISO 22000 internal audits. By focusing on critical control points and high-risk areas, organizations can allocate their time and resources more effectively.
As the ISO 22000:2018 documentation states:
"Risk-based thinking enables an organization to determine the factors that could cause its processes and its FSMS to deviate from the planned results, and to put in place controls to prevent or minimize adverse effects."
Foster a food safety culture of continuous improvement
Internal audits should not be viewed as a mere compliance exercise but rather as an opportunity for continuous improvement. Encouraging a positive attitude towards audits and involving employees in the process can lead to better results.
Regularly review and update the FSMS
ISO 22000 internal audits provide valuable insights into the effectiveness of an organization's food safety management system. It is essential to regularly review and update the FSMS based on audit findings and changing regulatory requirements.
As you monitor and measure internal food safety objectives, you'll quickly realize how you stack up against additional requirements that may be expected of your food business in the future. As you go through the ISO 22000 internal audit questionnaire, your record of answers where you do not conform will be a great feedback loop that you can use to improve upon and update the FSMS.
Download your ISO 22000 internal audit checklist today
Jump back up to the ISO 22000 internal audit template!
Whether you’re striving for ISO 22000 certification in the near future or just want to set your business in the right direction when it comes to food safety, this audit checklist will help set you up for success.
So we want to say kudos! While the ISO 22000 certification is not mandatory for food producers, taking the initiative to elevate your food safety standards is at least two-fold.
Doing this shows teams that leadership cares about and is continuously improving food safety culture and builds trust with existing and potential customers who use your food products (if you decide to share food safety practices externally, which you should).